Lead is very cheap and abundant; most importantly it is globally recycled about 99%
In our daily life, we are totally depending upon power whether it is an electric vehicle, mobile charger or laptop charger, they always carry a letter ‘V’ means Volt -the electricity unit named after the physicist Alessandro Volta. This physicist developed a first electric battery in the year 1800. Those days he was not aware that the whole world would be powered by batteries. after two centuries, we all know the importance of energy.
Energy can be generated from the burning fossil fuels (for e.g., coal) but it emits a large amount of CO2 and other harmful gases to the environment. Fossil fuels are also depleting with time and now the time has come to develop a carbon free technology. Now a days, people are moving towards renewable energy resources for e.g., solar energy, wind energy etc. It is expected that by 2050, to meet the global target of net zero greenhouse gases, almost 90% of global electricity will need to be generated from renewable sources. However, renewable energy is intermittent and weather condition dependent. Solar panels cannot generate power when sunlight is not available and if wind is not blowing then wind power cannot be generated. Growing demand for utilizing renewable energy motivates the development of energy storage devices, for both on-grid and off-grid applications.
For renewable energy storage, redox flow battery (RFB) is the best option because it has high efficiency, long cycle life, modular design and offer deep discharge. As compared to Lithium ion battery, RFBs are less likely to catch fire and lose less storage capacity even after thousands of cycles. Redox flow batteries (RFBs) convert and store electrical energy into chemical energy and release it in a controlled manner when required. RFBs differ from conventional rechargeable batteries in that the active material is dissolved in an electrolyte and it is circulated through the redox flow cells. The main advantage of RFB systems is decoupling power and energy densities in contrast to batteries and supercapacitors.
Several RFB technologies have been developed however, only few of them are commercialized. The most advanced commercialized RFB is Vanadium redox flow battery (VRFB). It was first developed in 1984 by Skylllas-Kazacos. At present, China is developing an 800 MWh VRFB stack. Other RFBs are not yet in application due to the high cost, maintenance, and other technical issues. Among the various RFBs, soluble-lead redox flow battery (SLRFB) is especially attractive as it employs single electrolyte, has no separating membrane; lead is globally recycled to about 99%. Although the membrane-less cell configuration bestows SLRFB cost-effectiveness over other flow batteries, there are challenges associated with the plating of PbO2, Pb dendrite formation and the presence of parasitic reactions. Since 2004, SLRFB is being researched and developed at laboratory scale but the technology is not yet commercialized.

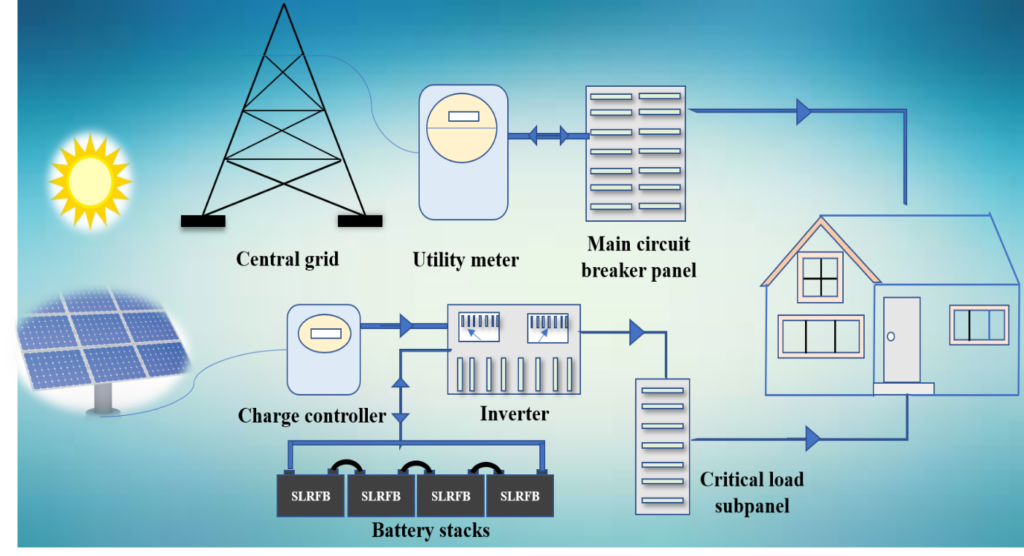
Image dictates the off-grid hybrid solar power system for homes
Working principle of SLRFB
In SLRFB, the electrolyte consists of Pb2+ ions dissolved in the methanesulfonic acid which is being circulated through the cell with the help of a pump. During charge, Pb metal is plated on anode side (negative electrode) and PbO2 deposition takes place at cathode side (positive electrode) resulting in a nominal cell voltage of 2 V. During discharge, both Pb metal and PbO2 come back to the solution as Pb2+ ions.
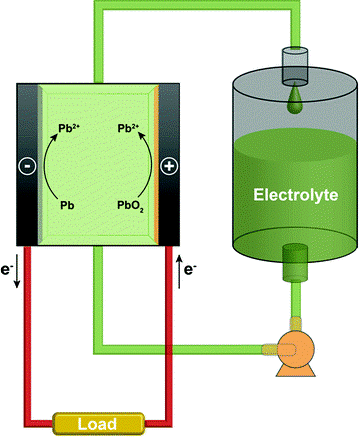
Image shows the working principle of SLRFB
Challenges in commercialization of the SLRFB technology
SLRFB shows better performance in terms of efficiency, and cycle life at lower current densities such as 20-25 mA/cm2. However, performance declines at high operating current densities. This happens due to problems associated with electrode reactions. The high overpotential at cathode (> 1.7 V vs. SHE) at higher currents leads to parasitic reactions such as O2 evolution on carbon electrodes. This reduces the coulombic efficiency of PbO2 deposition and consequently growth of Pb dendrites at anode and hence failing of the cell due to electrode shorting. Therefore, the cycle life the SLRFB is limited to 100-200 charge/discharge cycles with less areal capacity 20 mAh/cm2. At present, for the commercialization of the technology, it is necessary to increase the areal capacity (mAh/cm2) of the battery as well as operating current density either by modifying the electrolyte formulation, electrode modification or cell design.
Improved performance observed by IIT Madras Research Team
The main focus of the research in SLRFB is to increase the areal capacity. Prof. R. Kothandaraman research group at IIT Madras looking at reaching an areal capacity beyond 60 mAh/cm2 at high operating current densities (30-50 mA/cm2) with good cycling stability. In flow cell mode, the maximum cycles reported for SLRFB is about 100 and with maintenance, it was extendable up to ~300. Whereas, Kothandaraman group has increased the cyclability of the cell without any capacity decay for over 500 cycles not requiring any maintenance. With the modified electrolyte, even after 500 cycles, SLRFB exhibited an average coulombic efficiency and energy efficiency of 94%, and 74%, respectively. This is an important milestone in the development of the SLRFB and hence we believe it would be interesting for the flow battery community as it is a considerably cheaper system with excellent performance than the other redox flow systems.
For commercialization of the technology, our team is working to develop and demonstrate an 1kWh battery stack of SLRFB.
Author – Dr. Nandini Jaiswal, Institute Postdoctoral Fellow, Department of Chemistry, Indian Institute of Technology, Madras.