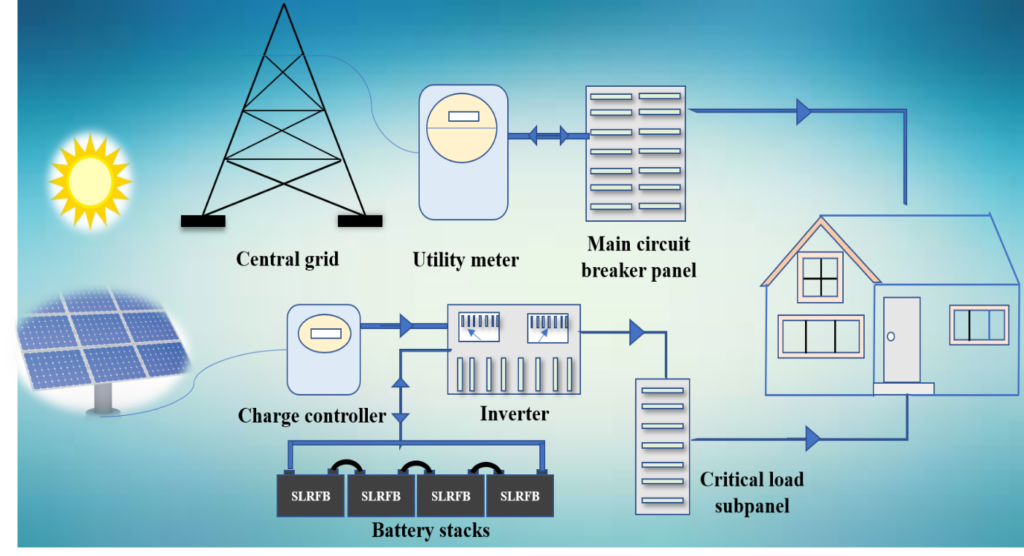
Due to the growing world’s population, the current energy consumption is ~ 580 million terajoules per year, according to The World Count. About 83% of this energy demand is derived from the fossil fuels causing deleterious effects on the nature & environment leading to global warming. Therefore, due to continuous depletion of fossil fuels and the increase of ecological footprint, renewable energy has become the keystone of power generation technologies. But renewable energy sources are not suitable for their direct integration with the grid as energy output from them is intermittent. This intermittency has provoked a need for safe, long-life, efficient, and cost-effective energy storage devices. Hydroelectric and closed loop off-river pumped hydro (CLORPH) are the proven energy storage solutions, since most of the river based hydroelectricity as options are exhausted and CLORPH and batteries are the attractive options. But combination of high initial cost and environmental concern blocks usage of CLORPH. Besides, assuming an energy consumption of 27GWh/day/million people, 400 m head (vertical distance between the reservoirs) and generation efficiency of 90%, nearly 1GL of water is required per GWh in the case of CLORPH, which is nothing but 27 kL/person. Whereas, assuming 60 Wh/L capacity of battery, only 450 L of electrolyte/person is required for flow battery operation. Therefore, batteries are the left out choice for the energy storage. Electrochemical energy storage systems (EES) such as redox flow batteries (RFBs) could buffer the intermittency and continuous fluctuation in power output, thereby seamlessly integrating the grid to renewable energy technologies. In addition to this, RFBs allows to isolate the power (cell stack) and energy (electrolyte tank). Since, the EES is envisioned for large scale storage of energy for long duration with low maintenance, the raw materials cost should be as low as possible giving rise to an system cost of < 75 $/kWh. Therefore, in this regard, lithium-ion batteries (LIBs), may not be an ideal candidate because the material cost is quite high especially long duration operation. Among various inorganic aqueous RFBs, vanadium redox flow battery (VRFB) and Zn-Br2 RFB are matured, but their capacious application for EES encounter with several techno-economical drawbacks. For example for VRFB, totally six materials are needed, which includes bauxite (for aluminium end plates), fluorspar (perfluorinated membrane), graphite for bipolar plates, phosphorous rock (electrolyte additive), and vanadium salt (electrolyte). Whereas, in the case of aqueous organic materials, the last two raw materials (phosphorous & vanadium) are excluded, therefore, reduction of critical raw materials is also one of advantages with aqueous organic redox flow batteries (AORFBs). The cost of VRFB electrolyte is around 80 USD$ kWh-1 and nearly 50% of the total cost of the battery is from vanadium. Thus, compared to inorganic RFBs, AORFBs could lower the levelized cost of energy storage as the raw materials involved is not limited by the geographical location of the project. Since India is global hub for pharmaceutical materials synthesis, the organic redox molecules with necessary functionalization can be easily synthesized in-house. In the recent reports, the electrolyte cost of few AORFB has been demonstrated to be less than 35 USD$ kWh-1 (based on half-cell estimation). In addition, AORFBs has several advantages over other aqueous RFBs such as; (i) organic redox active molecules are synthetically tunable to gain high oxidation/reduction potential, and solubility and thus high energy density RFBs, (ii) using of inexpensive, non-flammable, aqueous electrolytes consisting of inorganic supporting electrolytes KOH and NaCl etc. However, continuous degradation of the organic redox active molecules in harsh operating conditions is the primary concern for their commercialization. Thus, there is a need of developing low cost and efficient RFB technology to meet the growing energy storage demands. Therefore, we have attempted to demonstrate an aqueous organic redox flow battery with an inexpensive anthraquinone based compound.
Working Principle of AORFB
A schematic diagram of working principle of AORFB is shown in the Fig. 1. The system has two electrolyte reservoirs containing the negolyte and posolyte solutions (having active redox molecules), which is circulated to the electrodes by using pumps. The increasing concentration of active redox molecule and enlarging the volume of either negolyte or posolyte solutions are proportional to the energy density of the system. The energy density (Wh L-1) of a RFB system can be determined as:
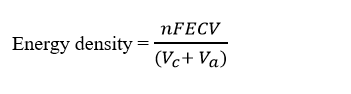
Where, n is the number of electrons involved in redox reaction, E is the cell voltage (V), F is a constant (26.8 Ah L-1), C and V are the concentration (mol L-1) and Volume (L) of the capacity limiting electrolyte (could be either catholyte (Vc) or anolyte (Va)). At IITM anthraquinone derivative in an aqueous alkaline medium was used as an anolyte with iron-based cathode redox couple yielding a cell voltage of 1.25 V per cell.
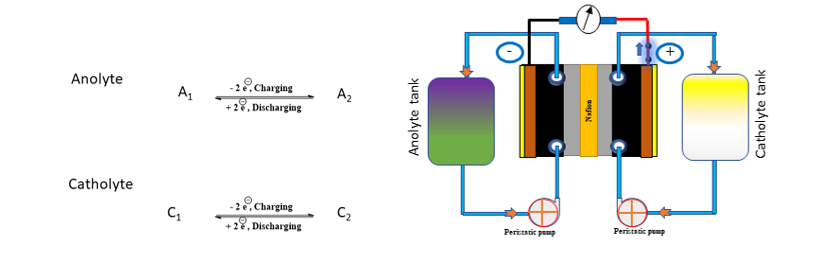
Fig. 1 Working principle of AORFB based on anthraquinone derivative
Challenges in the commercialization of the technology
Organic redox-active molecules are the better choice to build energy storage systems with lower levelized cost provided if they exhibit high solubility and stability/robustness during charge-discharge. Understanding how organic molecules degrade during the energy storage process is crucial to engineering structurally stable ones. Therefore, the main challenges with the AORFBS are less solubility of the redox active molecule and their stability, causing fast capacity decay.
Our group at IIT Madras has demonstrated an AORFB based on anthraquinone derivative with concentration ~ 0.7 M (nearly saturated solution) which could offer a capacity of ~ 40 Ah/L. In literature, often low concentration flow cell is demonstrated due to uncontrolled electrochemically inactive dimer formation at high concentrations. Our technology involves in avoiding this dimer formation therefore able to work with high-capacity anolyte. We have demonstrated at TRL 3 level and looking forward to reach TRL 9 with industry/startup funding
Author: Dr. Nandini Jaiswal, Institute Post-Doctoral Fellow, Clean Energy Lab, Prof. Kothandaraman Ramanujam Group, Department of Chemistry, Indian Institute of Technology, Madras