Carbon dioxide is a colorless and odorless gas present in the atmosphere. It is also called a greenhouse gas produced by burning fossil fuels (natural gas, oil, coal). It is a very stable and inert molecule with a high bond energy of around 750 kJ mol-1. Thus, high energy input is required to break the C=O bond.
Owing to the inertness of CO2, reduction of CO2 is becoming a challenging task. Catalysis is a process of conversion of CO2 to value-added products, eg. methane (CH4), carbon monoxide (CO), methanol (CH3OH), formic acid (HCOOH). Some of the catalysis methods for reduction of CO2 are thermocatalysis, biological fixation, electrocatalysis, and photocatalytic conversion, etc which can significantly boost carbon recycling and alleviate the consumption of fossil fuels.
Thermocatalysis: A thermochemical method converts CO2 to CO, methane, methanol, etc at high temperatures (500 °C and above) and pressure. It needs high energy cost and more heat energy is dissipated from the combustion of fossil fuels, which limits its industrial scale-up.
Biological fixation: In this method, microorganisms convert CO2 into biomass in the presence of sunlight and water. But the industrial scale is limited due to low bacterial loading, low electron transfer rate, and low efficiency of CO2 reduction. Also, the growth of these microalgae depends on various conditions like pH levels, and high temperature which further greatly affects the CO2 reduction process.
Electrocatalysis: This is a well-known technology where the conversion of CO2 is obtained by applying electrical energy using a catalyst. It requires high overpotential, and the choice of catalyst and electrolyte is necessary for high electrocatalytic performance.
Photocatalytic CO2 reduction: In recent years photocatalytic CO2 reduction technique has drawn significant attention for resolving the problem of global energy and environmental problems due to its excellent properties such as low cost, and environmental safety. It can transform and convert anthropogenic CO2 into valuable solar fuels such as gaseous (methane, carbon monoxide) and liquid products (methanol, formic acid, formaldehyde) by utilizing solar radiation as a sustainable energy source and a semiconductor photoactive material as a photocatalyst. It is also called “artificial photosynthesis,” which uses solar light and water to convert it into value-added chemicals and fuels.
Advantages of photocatalytic CO2 reduction:
Nowadays, climate change, global warming, and sea level rising have become serious issues for humans to survive. Excess combustion of fossil fuels produces huge amount of greenhouse gases (CO2) in the atmosphere. Hence to address these issues and to make a pollutant-free environment, there is a demand for some renewable energy sources that can capture this anthropogenic CO2 and easily convert it to valuable fuels. Solar energy is a natural, clean, and abundant energy source and an ideal renewable source for future fuel. Due to its intermittency, capturing and storing solar energy in the form of chemical bonds is much more effective for the reduction of CO2. The photocatalytic CO2 reduction method is one of the clean and sustainable technologies for the conversion of CO2 to solar fuels by utilizing solar energy with atmospheric CO2 and water, therefore solving the problems of global warming and environmental pollution.
This method can be performed at easy operating conditions, low pressure, and temperature, without applying any high energy. As a promising greener technique, it offers better resistance to catalyst deactivation and avoids all high-temperature reaction conditions.
Principles:
Since CO2 is a linear and thermodynamically stable molecule, it is imperative to search for an efficient photocatalyst that can effectively activate and convert CO2 to reactive species, producing valuable fuels. Generally, photocatalytic CO2 reduction comprises four steps:
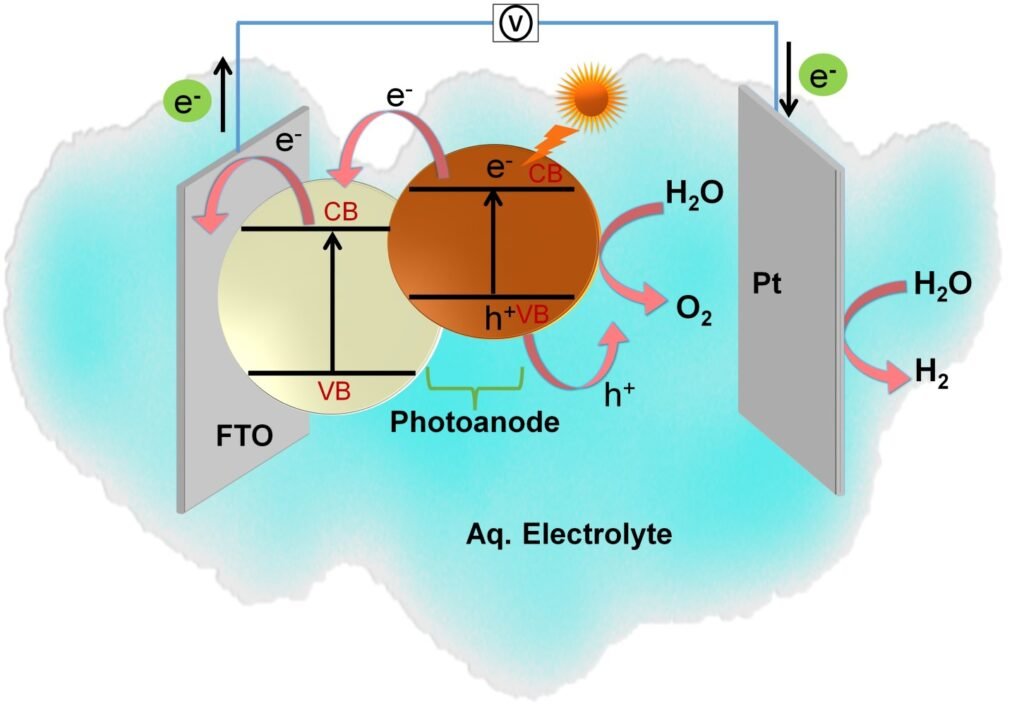
(1) Light absorption of semiconductor photocatalyst: semiconductors with a suitable band in the UV/visible region absorb solar radiation when exposed to a simulated light source.
(2) Generation and separation of charge carriers: the electrons are excited from the valence band (VB) to the conduction band (CB) of the semiconductor and leave holes in the valence band.
(3) CO2 reduction: photoexcited electrons in CB react with adsorbed CO2 to form products, and finally desorption of products from the catalyst surface.
(4) Holes present in VB move to the semiconductor surface and undergoes oxidation with water to form O2.
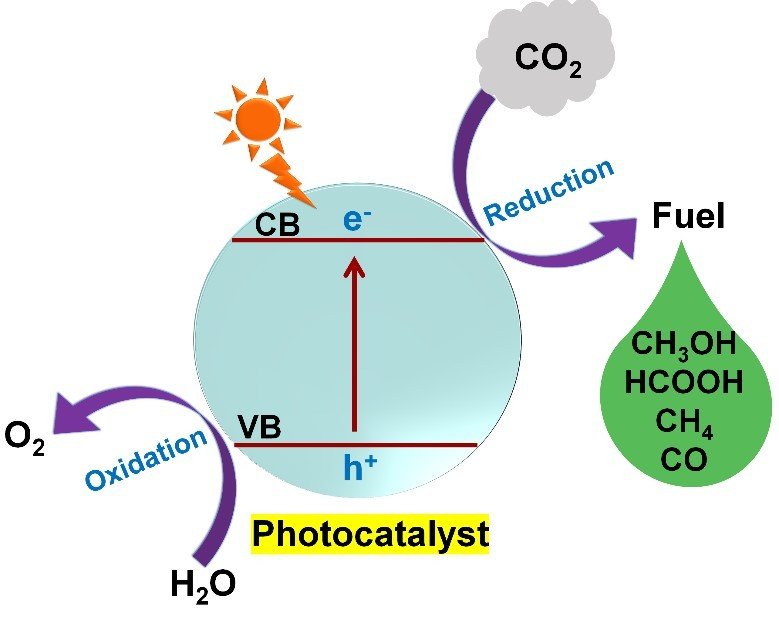
For photoreduction of CO2, the CB potential of semiconductor photocatalyst should be more negative than that of the corresponding CO2 reduction reaction and the VB potential of semiconductor should be more positive than that of the oxidation half-reaction.
Challenges:
Photo-driven CO2 reduction technology is an advanced method to mitigate the energy crisis and global warming problems. Though in photocatalysis photoactive semiconductors materials are employed to enhance the conversion process, it suffers from low CO2 conversion efficiency attributed to the limited absorption range of solar light, insufficient utilization of light energy, and slow electron transfer kinetic process. Moreover, the poor catalytic activity and stability of photocatalyst cause low selectivity of products, which further restrict their large-scale application.
For achieving a high conversion of CO2, the photocatalyst should have some excellent properties, eg. good light harvesting ability, high surface area, resistance to photocorrosion, and chemically stable. Sometimes, a single photocatalyst is insufficient to improve the photocatalysis process because of poor absorbing capability, rapid electron-hole recombination, low CO2 adsorption etc. So, one of the options to improve the photocatalytic performance is to couple a single photocatalyst with another photocatalyst with a suitable band gap (narrow band gap semiconductor). Moreover, cocatalysts with high surface area and more active sites could be the best solution to promote the conversion of CO2.
Considering the issues facing for CO2 reduction process, we at the solar energy research group are more focused on developing a robust photoactive semiconductor material for photocatalytic reduction of CO2. We are working on lead-free based perovskite material as a photocatalyst which are environment friendly and have the ability to absorb the light from entire visible to some part of NIR spectral region. We are also focusing on different strategies such as doping of transition metal ions, inclusion of cocatalyst and other semiconductors to enhance the photocatalytic CO2 reduction.
Author: Dr. Aparajita Das, National Postdoctoral Fellow, Solar Energy Research Group (SERG), Department of Chemical Engineering, Indian Institute of Technology, Madras