Metal-ion batteries, specifically, Li-ion batteries (LIB) are being realized as the next-generation battery technology due to their overwhelming advantages, including high energy density (270 Wh kg-1) and high cell voltage (3.6 V). However, safety is the major limitation for the conventional LIB because the explosion in portable devices such as laptop, PCs, smartphones, electric vehicles, etc. has been frequently reported due to the accidental fires in LIBs that originate from the flammable organic liquids used as the solvent electrolyte (SE). Therefore, replacing the volatile organic liquid with a solid electrolyte is necessary to realize safe LIBs. For this reason, all-solid-state batteries (ASSB) gained high attention over the past few years as the next-generation LIBs.
In ASSB, all cell components, i.e., anodes, cathodes, and electrolytes are solid. The electrode materials (both anode and cathode) employed for LIB are typically used as the electrodes for ASSB. The ionically conducting inorganic materials (oxide-based and sulfide-based) are used as the solid electrolyte (SE). The SE can be either inorganic or polymeric in nature or altogether. An ideal Li containing SE should possess the following characteristics of, 1) high ionic conductivity (>1 mS cm-1) at room temperature, 2) intimate contact with solid electrodes, 3) the ability to operate over a wide electrochemical window, and 4) good compatibility with Li or other anode materials. Considering the above facts, several SEs have been developed and studied. However, a few more challenges still remain, that need to be addressed to accelerate the commercial application of ASSB are:
1. Development of high-capacity solid electrodes (anode and cathode);
2. Fabrication of ASSB cell with the stable electrode-electrolyte interface;
3. Construction of ASSB cell with high-rate capability and cycle life.
So far, the ASSLiB research field is dominated by the development of SEs and cathodes, while the development of anode materials has been hardly explored, and the research efforts are still at an early stage.
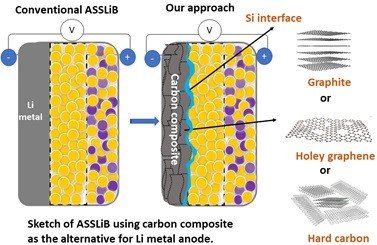
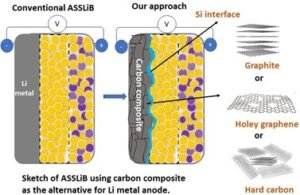
Li metal holds a high theoretical capacity (3860 mAh g-1) and low redox potential for Li storage (-3.04 V vs. RHE), owing to that, in many cases, Li metal being used as the anode for ASSLiB. However, Li metal has poor compatibility with solid electrolytes (both sulfide and oxide based) due to its high reactivity, leading to dendrite formation and causes of battery failure. Considering the reactivity of Li with SE, dendrite growth, and the expenses for surface protection, Li metal is not a material of choice as anode active material for ASSLiBs. Therefore, the practical use of high-capacity anodes is imperative to increase the energy density of the ASSLiBs, not only because of their high capacity but also to avoid internal short circuits due to Li dendrites. Similar to the LIB, graphite was attempted to use as the effective anode for ASSLiB. However, in some cases, graphite shows poor compatibility with solid electrolytes leading to fading in capacity, thus, graphite is not preferred to use as the state-of-the-art anode for ASSLiB.
Our recent study (https://doi.org/10.1021/acs.jpcc.2c04024) shows that graphite can deliver a capacity of 274 mAh g-1 (equivalent to 0.7 mAh cm-2), though there is a severe fading in capacity. On the other hand, the cell with Sn/graphite (Sn-17wt.%, graphite-83 wt.%) anode electrode shows a discharge capacity of around 470 mAh g-1 (equivalent to 1.8 mAh cm-2), which is significantly high compared to graphite. The obtained capacity is reasonable but insufficient for high energy density battery applications. Moreover, the applied current is 0.2 mA cm-2 which is extremely low and nearly four times below the range required for electric vehicles (1 mA cm-2).
Our research works at IIT Madras intends to develop high-capacity electrode materials, especially Si with graphite for ASSBs and suitable solid electrolytes using Li+ as transferring ions. In addition to the material development, our research efforts will focus on cell configuration, i.e., fabrication of cells with low internal resistance, optimum applied pressure (<370 MPa), the smaller thickness of solid electrolyte (SE) separator, low consumption of SE for making anode composite, and the structural engineering of electrode for the effective Li-ion diffusion. Most importantly, our research will aim to understand fundamental challenges associated with ASSB studies, including volume changes of electrodes (anode and cathode), the mechanism for Li+ storage under solid-state configuration, gas evolution, and identification of byproducts formed at the electrode-electrolyte interface.
Author – Dr. Palani Selvam, Assistant Professor, Department of Chemistry, Indian Institute of Technology, Madras