Hydrogen is the most abundant element in the universe, greater quantity in water. It is considered as a holy grail in the energy community. It is an advanced renewable energy source and carrier produced from other energy sources. It also offers a high value of gravimetric energy compared to gasoline (i.e., 120 MJ/kg versus 44 MJ/kg) and is regarded as one of the clean fuels with no greenhouse gas emissions, that can be channelized with a fuel cell to produce electricity and water. The main advantage of hydrogen as a fuel is that it is non-toxic, does not cause any corrosion, and is environmentally friendly. Thus, this fuel can be utilized in electric devices, aircraft, shipping, etc.
Various technologies generate hydrogen fuel, including steam reforming/oxidation of partial hydrocarbon, coal gasification, and water splitting. Except for water splitting, other techniques utilize fossil fuels and emit more amount of greenhouse gas CO2 which creates serious environmental issues. Whereas, water splitting is a simple method that uses renewable sources such as water, hence not causing any environmental problems. Hydrogen production through water splitting now has become more fascinating as it is based on the dissociation of a water molecule. The water splitting technique is mainly comprised of various categories such as electrochemical, photoelectrochemical (PEC), photocatalytic, photobiological, thermal decomposition, etc. Electrochemical water splitting employs direct electricity to split water whereas Photobiological water splitting is similar to photosynthesis which generates a very less quantity of hydrogen with the aid of an algae bioreactor. Thermal decomposition requires a higher temperature for this process and yields a very low amount of hydrogen. Thus, both Photocatalytic and PEC water splitting offers a significant avenue for the sustainable generation of hydrogen fuel. But the main drawback of the photocatalytic part includes difficulty in the separation of products- hydrogen (H2) and oxygen (O2), thereby reducing the efficiency. On the other hand, in PEC water splitting, pure quality of H2 and O2 can be separated by using a suitable photocathode and photoanode.
Advantages of PEC water splitting
PEC water splitting is a promising pathway to convert solar energy to hydrogen with high conversion efficiency as the only required energy source is solar energy, which is renewable, continuous, and inexhaustible. The raw material used for the PEC is water, which is a renewable natural resource, and abundant. A small reaction potential (1.23 V) is required to execute such process. This technique is simple, cost-effective, and substantially less expensive than gasoline. It is a sustainable and pollution-free energy conversion process for hydrogen energy generation.
Photoelectrochemical (PEC) water splitting
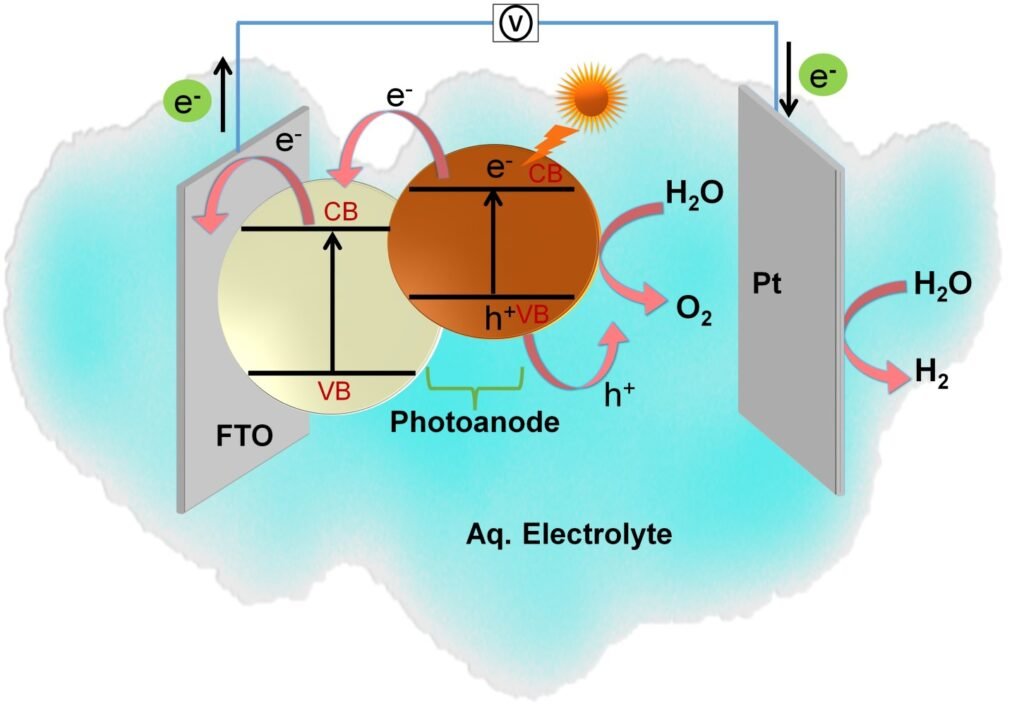
Solar radiation is the most abundant, accessible, and clean energy source. Due to its intermittent nature, it is imperative to capture and utilize this energy with improved technology. One of the interesting approaches is to use solar photons to drive chemical reactions uphill and store their energy in chemical bonds. PEC water splitting often called solar water splitting is a technique that has garnered wide attention in the conversion of light energy to H2 and O2 by splitting water in a photoelectrochemical cell using semiconducting active electrodes. We can call this generated hydrogen as “green hydrogen” because it is produced via a renewable energy source like solar energy.
The PEC cell involves two or three electrode configurations, immersed in an aqueous electrolyte. One of the electrodes should be made of a semiconductor material that can be able to absorb light radiation. The process of PECs is based on the following ways:
(1) photoexcitation in the semiconductor photoelectrode under light illumination, (2) charge separation between photogenerated electrons and hole pairs, (3) holes at the surface of photoanode undergo oxidation and releases oxygen (Oxygen evolution reaction (OER)), and simultaneously photoexcited electrons transfer from photoanode through an external circuit to the surface of the cathode, undergo reduction to form hydrogen (Hydrogen evolution reaction (HER)). In this PEC cell, the potential required to break water molecule is 1.23 V. The water oxidation and reduction reaction can be attained via PEC water splitting at potentials below 1.23 V and above 0 V compared to the reversible hydrogen electrode (RHE).
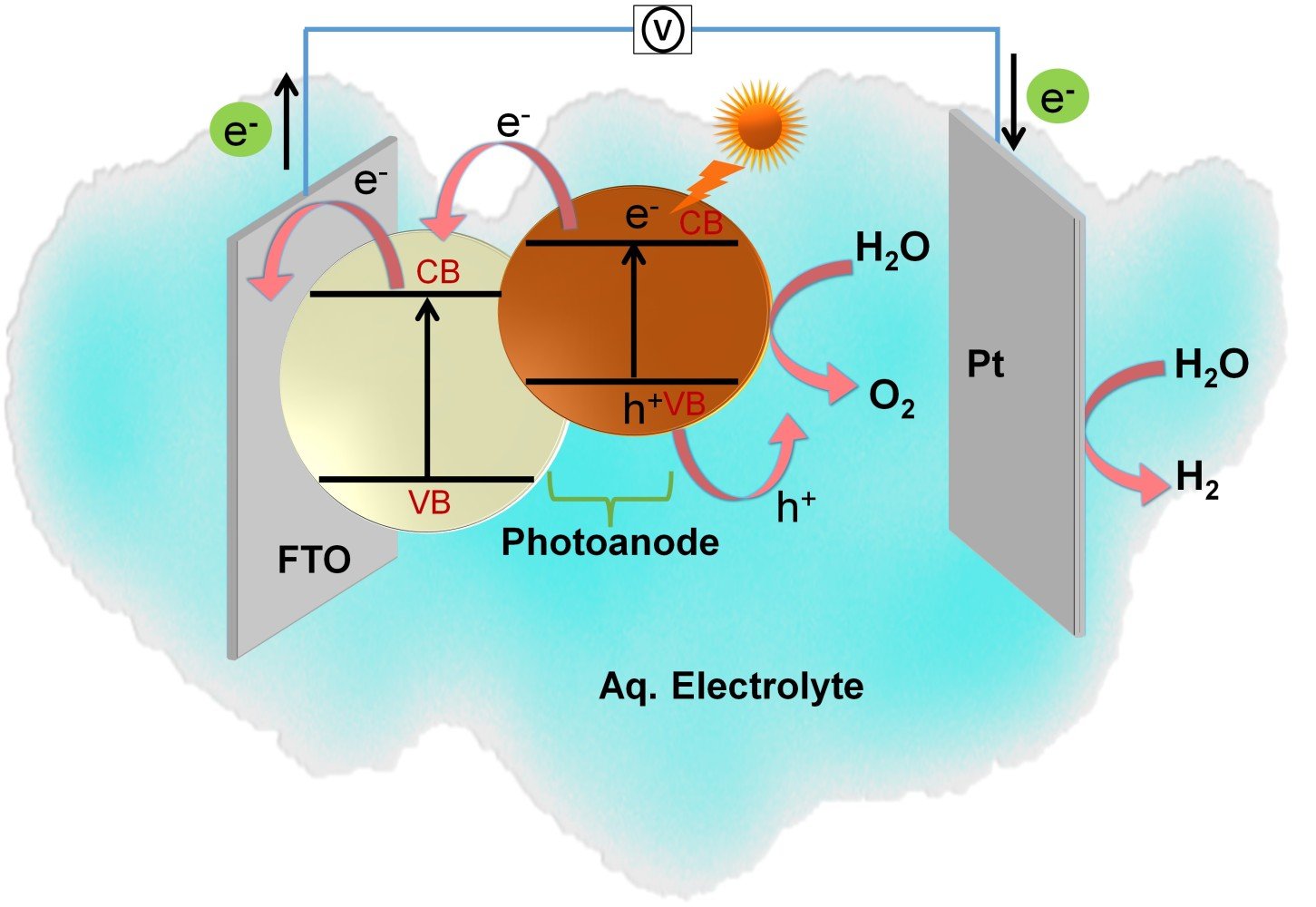
Schematic representation of PEC water-splitting reaction.
The semiconductor photoelectrode plays a pivotal role in achieving an effective PEC water splitting reaction. These materials should be non-toxic, low-cost, easy to process, and highly conducting. They should harvest the entire visible spectrum and exhibit long-term stability in an aqueous electrolyte medium. Also, they should be able to generate sufficient voltage to split water upon illumination and facilitate charge transfer at the electrolyte/electrode interface.
Challenges towards the PEC water splitting
Solar hydrogen production via PEC water splitting is a propitious energy conversion approach. Still, it is challenging for PEC technology to make it practical and commercially viable. High production cost, long-term instability, and limited light-harvesting properties in semiconductor photoelectrodes need to be resolved. During the water splitting reaction, there may be an energy loss, which is primarily attributed to fast charge recombination at the electrolyte/electrode interface. Thus, to achieve a high solar to hydrogen efficiency, the semiconductor materials should be narrow band semiconductors and more robust against photo-corrosion. A suitable band position must be applied to the proper oxidation and reduction half-reaction. Also, we have to be more focused on fabricating the photoactive electrode with low cost, high efficiency, and good durability.
To overcome such challenges of PEC water splitting, the “Solar Energy Research Group” group is trying to develop the best photovoltaic materials capable of splitting water into H2 and O2. Our main research focus is to minimize the use of fossil fuels to make a net-zero emission. For the first time, we have introduced a new non-toxic vacancy-ordered double halide perovskite material (A2BX6) for this application. Compared to conventional ABX3 type halide perovskites, A2BX6 type materials exhibit better stability and lower toxicity respectively. Such perovskite materials absorb entire visible light and are extremely stable in all pH media. They are also stable in ambient conditions for more than 2 years. In our lab, we have developed a vacancy-ordered perovskite material, ‘Cs2PtI6’, which displays superior stability in aqueous solutions of extremely acidic and basic pH medium. They are also stable to prolonged exposure to ambient conditions and high temperatures. Cs2PtI6 has been employed as the best electrocatalyst for the hydrogen evolution reaction and a photoanode for the water oxidation reaction. For PEC water oxidation, Cs2PtI6-based photoanode showed the photocurrent of 0.8 mA cm-2 at 1.23 V (vs. RHE) under 1 sun illumination.1 By combining a halide perovskite-based photoelectrochemical device with solar light, we have successfully split water into hydrogen and oxygen.
Author: Aparajita Das, National Postdoctoral Fellow, Aravind Kumar Chandiran, Assistant Professor, Department of Chemical Engineering, IIT Madras.