We can learn many things by observing nature. She is the best teacher, so why not go back and ask her to hint about how to fix a serious issue that we are all concerned about right now- Carbon dioxide.
Coal, Oil, and Gas- A Historical Outlook
All biological systems like humans, animals, plants, and microbes are primarily composed of carbon, oxygen, and hydrogen. These creatures, when buried, experienced high pressure and temperature conditions under the soil for millions of years. Based on the location and the chemical composition of these fossils, eventually, they got converted into either solid, liquid, or gas. These solids are what we find as coal now. The liquids and gases are crude oil and natural gas, respectively. These are the primary fuels that we use in various vital applications such as manufacturing and transport and are known as fossil fuels. Apart from playing a crucial role in supplying energy, they directly or indirectly dictate how politics and policies play around various nations (but this is a story for later).
What is a Fuel, and why does it cause CO2 emissions?
In rough words, fuel is any substance that can emit energy when reacted with other substances. The energy stored in fuels as chemical bonds gets released while combusting them. This energy can be used in the applications described above. Typically, we burn the fuel in the presence of oxygen, and this ends up producing by-products along with energy. Since all these fuels are built by carbon primarily, its reaction with oxygen produces CO2 as the primary by-product. Other emission products, such as CO and NOx, are out of the scope of this article.
Wasn’t there any CO2 before we started to exploit fossil fuels?
CO2 has always been a part of ambient air. Decomposition, respiration, and ocean release are the “natural” sources of CO2. Then how were the CO2 levels kept in control for centuries? The answer is photosynthesis. It is critical to note that CO2 is not a toxic gas, and it is a vital part of the carbon cycle that facilitates life on this planet. In other words, without CO2, life wouldn’t be possible on earth. The plants/trees on this planet were sufficient to capture the excess ambient CO2 and water and convert them into starch and oxygen with the help of sunlight. However, now we have more CO2 (thanks to the increased usage of fossil fuels) and fewer plants/trees than before that could capture and utilize the CO2 in photosynthesis, leading to the accumulation of CO2 in the atmosphere.
What does photosynthesis teach us?
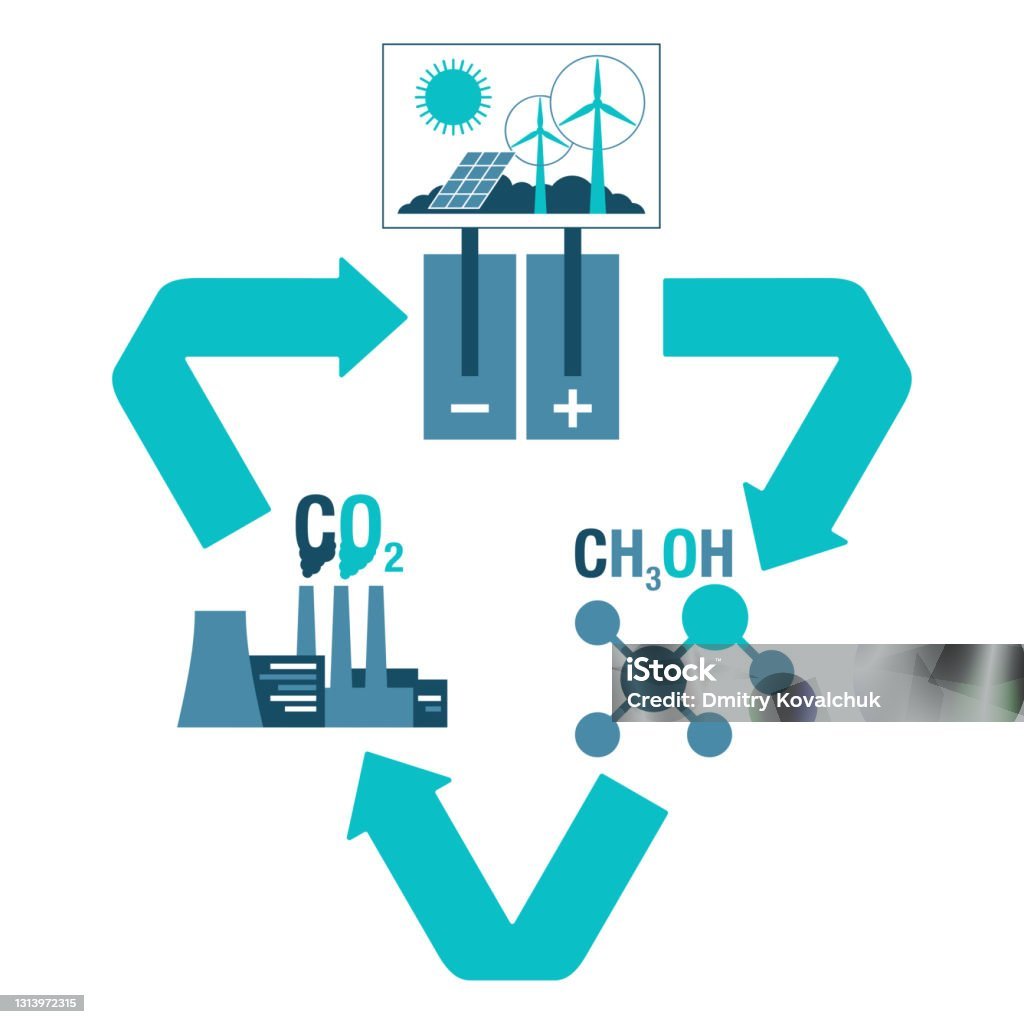
In photosynthesis, plants capture CO2 and upgrade it to starch (a useful chemical) using water (the source of hydrogen) and energy. In short, CO2 is used to produce a valuable substance. What if we try to emulate this process in the laboratory? Most of the day-to-day products we use are made up of carbon. Why not use the excess flue CO2 produced to produce such products? Almost every fuel we use is made up of carbon. We can also produce fuels from CO2 and use them as an energy carrier for various transport and industrial applications. Take a look at the below equations (this is not a typical scary textbook chemical equation).

Carbon dioxide + Water → Starch (Natural process (Photosynthesis))
Carbon dioxide + Hydrogen (which comes from water) → Useful products (Artificial process)
The simplified chemical equations clearly emphasize the lesson nature gives us on utilizing and managing CO2. The useful product could be anything ranging from methane, methanol, CO, syngas, dimethyl ether, formic acid, formaldehyde, higher hydrocarbons, aromatics, plastics, and so on, depending on the catalyst and the reaction conditions.
For instance, Carbon Recycling International (CRI) is an Iceland-based company that produces methanol from CO2 captured from a coal-fired power plant. While the power plant provides the country with electricity, the methanol produced from the power plant’s emissions (they call it Vulcanol) is used as a fuel in automobiles, closing the cycle1. This process of capturing and utilizing CO2 is an attractive research area and an integral part of our CCUS (Carbon Capture Utilization and Storage) laboratory.
What are the significant challenges involved in achieving this?
Any chemical reaction will have two significant challenges- sourcing raw materials and ‘selectivity’ towards the desired products. The two raw materials we require for this process are carbon dioxide and hydrogen.
Although CO2 is found in excess (which is why we are trying to reduce it), it is challenging to capture CO2 from various industrial and automobile sources and separate them, as they are usually found with other impurities such as sulfur and nitrogen oxides. Different separation techniques such as adsorption, absorption, membrane separation, and cryogenic separation are researched to separate CO2 from this mixture.
It is also worth noting that CO2 is a highly stable molecule, making it difficult to undergo chemical changes. Hence various new catalysts and novel reactor configurations are being developed to make CO2 react to form valuable products.
The next major challenge is the origin of hydrogen. Hydrogen is categorized with various colors based on its source (details to follow in the upcoming articles). In short, if hydrogen is obtained from a carbonaceous source such as coal, it is usually given the color grey/brown, meaning that they are not very environment-friendly as they are accompanied by the emission of CO2 and other greenhouse gases. However, if we could produce hydrogen from water splitting (as we learned from photosynthesis) using renewable energy, it would be called “Green Hydrogen”. Although this process is environmentally friendly, the economics of green hydrogen production would determine the feasibility of this process. Hence, optimization of water electrolysis (the process of separating hydrogen from water) becomes a significant research area.
A few reaction pathways use compounds such as ammonia (NH3) and methane (CH4) as the source of hydrogen. There are also strategies where electrochemical or photochemical or a combination of both can generate hydrogen. We will discuss these approaches in detail in our upcoming articles.
Additionally, there is a challenge in obtaining selectivity. Since carbon dioxide and hydrogen could react to produce many different products, it is essential to deduce the suitable catalyst and define the reaction conditions at which a particular product would form. For instance, CO2 and hydrogen produce methane (a gas) on nickel-based catalysts at atmospheric pressure, whereas methanol (a liquid) on copper-based catalysts at elevated pressures. Hence, understanding these reaction systems is crucial to designing appropriate catalysts and driving the reaction in the desired direction.
These are a few among the diverse research directions we are working on in our CCUS laboratory.
What is the future of such CO2 utilization projects?
CO2 emission occurs predominantly from cement, iron and steel, and coal-fired power plants. These sectors contribute significantly to a country’s economy and couldn’t be overlooked. Hence, we can source vast amounts of CO2 from such industries for producing valuable chemicals. We can also think of integrating CO2 utilization plants with these emitters so that they can have the option of making useful chemicals/fuels from the waste CO2 they generate. If we synthesize fuels such as methane and methanol from waste CO2, they can be used back as fuel in the same industry, reducing their dependency on coal (also reducing its pulverizing cost). Lesser coal would correspond to lesser mining costs and easier waste handling, as liquid and gaseous fuels don’t produce ash, unlike coal. Commercialization of such fuels in the manufacturing and transport sectors would also enable us to minimize foreign dependence on oil, placing us much better economically.
1https://www.carbonrecycling.is/
Author- Ajay Koushik V, Research Scholar, Indian Institute of Technology, Madras