Societal reforming involves breaking and restructuring existing systems to create positive societal change. This reformation can happen through several routes, such as policy, education, economics and cultural understanding. Moreover, a practical societal reformation might demand a willingness to challenge the traditional/conventional ways of thinking. This reformation would eliminate the notorious and harmful societal elements and pave the way for a peaceful and equitable society. In social reforming, the goal is to convert outdated systems and structures into more efficient and effective ones, which can be used to create a better society.
As the name suggests, methane reforming is very similar to the above-discussed societal reforming. Technically, it is the process of breaking down methane, a notorious greenhouse gas, into a valuable mixture of hydrogen and carbon monoxide known as synthesis gas (or, shortly, syngas). This process is endothermic, meaning it needs some external energy to make it happen (similar to the willingness required to bring up a societal change). Syngas is valuable for two important reasons: (i) It contains hydrogen, and (ii) It is the building block for many organic chemicals. With the suitable catalyst, reactor and operating conditions, syngas can be converted into any hydrocarbon (or even some other organic compounds such as methanol). This versatility makes syngas an industrially important chemical which in turn signifies the importance of methane reforming.
Let’s superficially look into the chemistry behind methane reforming to understand how it is done. We have methane (CH4) that needs to be transformed into carbon monoxide (CO) and hydrogen (H2). This means that we need to add some component that has oxygen (O). What are the first three compounds that come to mind when reading the last statement?- Yes! You got it right!- They are water (H2O), carbon dioxide (CO2) and oxygen (O2).
Using water (technically steam) in methane reforming is called “Steam Reforming”. It is one of the most common forms of reforming, constituting about 95% of the total hydrogen production in the world. This form of reforming has been in practice for about a century and hence is well-known. Since both methane and steam have hydrogen, it produces syngas of a very hydrogen content. This is one of the challenges with this process; most chemical synthesis doesn’t need syngas of a very high hydrogen content (H2/CO~3-3.5). However, if hydrogen production itself is the goal, this might be the best form of reforming, only considering hydrogen yield as the target. Moreover, steam production is an energy-intensive process and steam methane reforming in itself is energy intensive.
Let’s now look into the next oxidant: CO2. This process of using CO2 as an oxidant to produce syngas is called “Dry Reforming”, and it has a dual advantage. At the cost of producing valuable syngas, we use up CO2, a greenhouse gas. Hence, this is an effective carbon capture utilization and storage (CCUS) technique. Also, the reactants of this reaction are methane and CO2, both greenhouse gases. Unlike steam reforming, the oxidant doesn’t have hydrogen in this reaction. So, the syngas produced through this process has very low hydrogen content (H2/CO~1). Although it was said in the previous paragraph that a high hydrogen content is not desirable for several chemical syntheses, a syngas of this ratio (around 1) is too low for any chemical process, which is the major drawback of dry reforming. Also, since it uses up methane and CO2, a lot of carbon is deposited on the catalyst surface, decreasing the lifetime of catalysts (and increasing the capital cost).
In a nutshell, steam and dry reforming processes are energy intensive and produce syngas of different qualities. Steam reforming produces syngas with very high hydrogen, whereas dry reforming produces syngas with very low hydrogen content. Steam reforming is carbon positive (i.e., lots of CO2 is emitted for and during the process). In contrast, dry reforming is carbon negative (or at least carbon neutral) as CO2 is the critical reactant in the process. Before we discuss insights on how these drawbacks can be overcome and how this process can be optimized, we will look into one more oxidant that could be used for reforming: oxygen.
Methane is a potential fuel, and adding oxygen would result in methane combustion. When methane completely burns, it produces lots of energy accompanied by the emission of CO2. However, in the context of reforming, we need to produce syngas. To achieve this, methane and oxygen should be added to the same reactor, and the process should be terminated before it proceeds to completion. In other words, we need to conduct controlled combustion of methane and deficit oxygen presence, and this process is called “Partial oxidation” of methane. This produces slightly higher hydrogen content syngas than the dry reforming process, and lower than the steam reforming process. Unlike steam and dry reforming processes, this process is exothermic, meaning this reaction will release heat. But since this process is a controlled form of combustion (in some rough sense), this will be carbon positive. One major disadvantage of this process is that they can produce hotspots in the reactor (due to the released heat), and there will be severe safety issues if the reaction is not appropriately controlled.
The below table gives a summary of the discussion above.
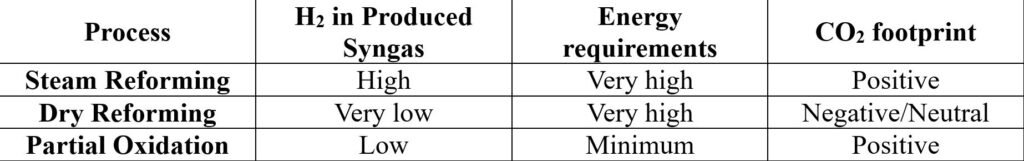
As seen in the table, all of these processes have a bunch of positives and negatives. Now let’s get to the interesting part. The exciting thing about recent research in these reforming processes is that it need not have just one oxidant. There can be more than one oxidant that can be fed along with methane to overcome these drawbacks.
Let’s discuss all possible combinations in the below paragraphs:
“Autothermal Reforming” is a combination of steam reforming and partial oxidation of methane. As the name suggests, the reaction is self-sufficient in terms of energy requirement. We discussed earlier that steam reforming is energy-intensive, and partial oxidation is exothermic. Hence, by combining these two processes, we achieve a balance. The energy required for steam reforming will be provided by the partial oxidation of methane within the same reactor. Additionally, the quality of syngas here will be an average of the quality of syngas produced by the individual process. Despite a few visible advantages, this process ends up emitting more CO2. “Autothermal Dry Reforming” is the process of combining dry reforming and partial oxidation of methane. This process has advantages in decreasing energy requirements, as discussed above. Additionally, this process reduces the coke formation on the catalyst by burning the solid carbon, which in turn gets consumed in the reaction. However, this process produces syngas with lower hydrogen content and cannot be the first choice if hydrogen production is the primary objective.
“Bi-reforming” is the combination of steam and dry reforming. This process can produce syngas of the desired quality due to the flexibility in the feed ratios. In other words, one has the flexibility to choose how much steam and carbon dioxide can be used for reforming. If the objective is to produce syngas with a very high hydrogen content, then there can be more steam and less CO2. On the other hand, if CO2 utilization is the target, then more CO2 can be used in the feed stream. However, since both of these processes are endothermic, bi-reforming requires a lot of external energy. The final form of the reforming process is called “tri-reforming” of methane. As the name suggests, it uses all three oxidants at a time. It is like the “Avengers” of the reforming process. All three oxidants have their advantages and disadvantages. This process is highly flexible and can be optimized based on one’s objectives/target. If producing high-quality syngas with reduced energy consumption is the goal, then more H2O and O2 can be sent as feed accompanied by some CO2 that would account for CO2 produced in the reaction. If maximizing CO2 utilization at minimized energy input is the target, the feed stream would have more CO2 and O2 with some H2O to enhance the syngas quality.
Overall, this is a multi-objective optimization problem with conflicting objectives. For instance, if one needs low coking, the energy input would be higher and vice versa. We have the following levers that can be used for controlling the reaction:
More CO2 in the feed- To maximize CO2 utilization
More H2O in the feed – To produce syngas with higher hydrogen content
More O2 in the feed – To decrease energy input
The choice of oxidant also depends on the availability of these components at a given location.
The potential objectives of the process could be as follows:
1. Optimal H2/CO ratio in the syngas
2. Maximum CH4 conversion
3. Maximum CO2 utilization
4. Maximum Energy Efficiency
5. Low Coke deposition on the catalyst
In conclusion, several reforming processes were discussed, and their advantages and shortcomings were reported. This analysis is being done at our CCUS laboratory at multiple levels. As discussed in the previous article, a complete multi-scale analysis of the problem would give interesting and exciting results. Due to the complexity of the problem, there is a scope for optimization at multiple levels: the choice of catalysts, the choice of the reactor, the reactor operating conditions, and the plant design and control. Since the objectives of this process are conflicting, there is no single best solution to this problem. One needs to optimize all the layers mentioned above and see which reforming process at which feed composition (also considering the availability factor) and reactor condition best suit one’s needs.
Author – Ajay Koushik V, Research Scholar, Kinetics and Catalysis Research Group, Indian Institute of Technology, Madras.



