It is a shocking fact that carbon emissions have been increasing from 5 GtC/year to 9.9 GtC/year in the period of the last 40 years. There are prominent factors like the conversion of forest land use for agricultural land, urbanization, and deforestation resulting in carbon transfer from land to the atmosphere at the rate of ~1.5 GtC/year. To counter the adverse impact of increasing carbon dioxide levels in the environment, research is going on in the field of carbon sequestration. The operation will be on the order of tons of gigatons of CO2 per year if CO2 sequestration will not considerably reduce atmospheric carbon.
Sequestering CO2 as gas hydrates
Clathrate hydrates, also known as gas hydrates, are non-stoichiometric solid compositions comprised of water molecules that, at the right temperature and pressure, form cage-like crystal structures that enclose the guest molecules. Water molecules use hydrogen bonds to build the hydrate lattices. One guest molecule of the right size, such as CO2, can typically fit into one cage and stabilize the structure. One unit volume of hydrates can theoretically hold up to 184 unit volumes of gas (under the STP condition). The possibility of employing CO2 hydrates as a storage form for CO2 sequestration is suggested by the abundance and widespread dispersion of CH4 hydrate in nature.
Approaches of CO2 sequestration as clathrate hydrates

The numerous methods for storing CO2 as clathrate hydrates are shown in Figure 1. CO2 sequestration can be delivered to the storage sites after being captured from the emission sources. Deep oceanic basins, sub-seafloor saline formations, depleted natural gas hydrate reservoirs, existing natural gas hydrate reservoirs (through CO2-CH4 exchange), subpermafrost regions with unfrozen water, and depleted oil and gas reservoirs partially saturated with water are examples of potential CO2 storage sites related to CO2 hydrates.
[1] CO2 Storage As Hydrate in Seawater
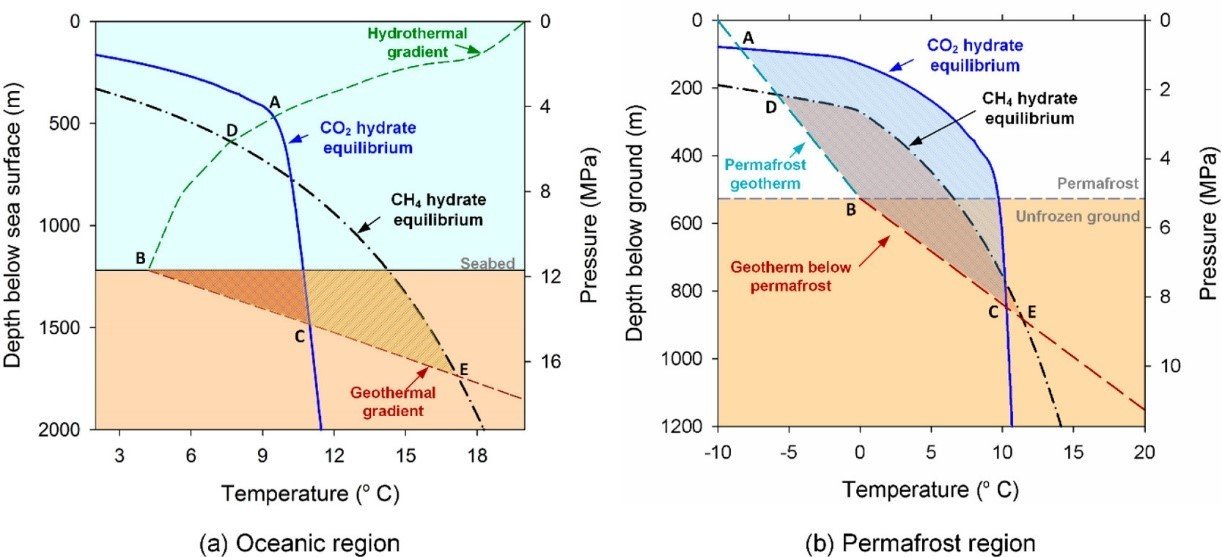
Ocean heat gradient and depth both affect the thermodynamic stability of CO2 hydrates in marine environments. In Figure 2, the phase equilibrium of CO2 hydrates is shown superimposed on the ocean’s hydrothermal gradient and the seafloor’s geothermal gradient, which is assumed to be at a depth of 1200 meters for the sake of this illustration. The first field CO2 injection experiment was carried out in 1999. 80 In the hydrate stability zone, small injections of CO2 (up to a few liters) were made into the water column surrounding the sea floor in Monterey Bay, California, at depths ranging from 349 m to 3627 m.
In addition to producing CO2 hydrates in the ocean, it was also suggested that CO2 hydrates might also be generated on land, transported by ship, released into the ocean, and then sunk to the seafloor. A higher density than seawater can be achieved by creating compact CO2 hydrate blocks. Successful CO2 hydrate composite particle production and sinking behaviour are encouraging for both methods. It is crucial to address the stability of these CO2 hydrates in the ocean environment, particularly when an ocean current is present to disturb them, as well as their effects on the marine ecosystem. Most field experiments to yet have only been conducted at water depths of up to 4 km.
[2] CO2 storage as hydrate in permafrost regions
In addition to coastal environments, permafrost regions are a common habitat for natural gas hydrates. While methane hydrate resources in permafrost regions are often predicted to be nearly two orders of magnitude less abundant than those in marine regions, they are nonetheless thought to be the most recoverable natural gas hydrates due to fewer technical difficulties and lower costs. Similarly to this, the permafrost zone may make it easier to store CO2 as hydrates. With geothermal gradients of 1.9°C/100 m for the permafrost layer and 3.2°C/100 m for the unfrozen layer, respectively, Figure 2 shows the CO2 hydrate stability zone in a hypothetical permafrost location. The depth between points A and C is covered by the stability zone of CO2 hydrates.
The best locations for storing CO2 have been said to have a continuous permafrost layer as a sealing cap, a domal permafrost base, and no taliks (regions of partially frozen ground surrounded by permafrost). However, in actuality, permafrost layers frequently include fissures and warm patches, thus it is important to carefully consider the probability of CO2 escape. The thickness of the CO2 hydrate stability zone varies from 50 to about 800 meters depending on the region due to the permafrost thickness and geothermal profile. It is recommended to store CO2 in the subpermafrost reservoirs because the frozen rocks in the permafrost layer have low permeability.
[3] CO2 Storage as Hydrate under the Seafloor
To avoid being disturbed by ocean currents and then provide a more confined environment, CO2 hydrate formation beneath the seafloor may be preferable to storing CO2 in seawater. The potential storage locations are the extensive saline formations beneath the ocean floor, which may include water-bearing formations including permeable rocks, rock fissures, and unconsolidated materials (gravel, sand, or silt). The upward migration of CO2 to the seafloor may theoretically be doubly constrained by the hydrate cap and gravitational confinement.
While the aforementioned theoretical studies demonstrate the viability of sustained CO2 storage as hydrates beneath the seafloor, the actual work to date is primarily restricted to laboratory experiments. More experimental work is required to fully comprehend the hydrodynamics, thermodynamics, and kinetics of the multiphase and multicomponent flow problem related to CO2 injection, hydrate formation/dissociation, and other potential chemical reactions. Furthermore, field testing must yet be carried out to show that large-scale CO2 hydrate storage beneath the seafloor is feasible.
[4] CO2−CH4 exchange in methane hydrates
Injecting CO2 or CO2-containing gas into the natural gas hydrate reservoirs can also be used to store CO2 while simultaneously producing methane. The CH4 and CO2 hydrate stability zones for a fictitious marine region and a permafrost location, respectively, are plotted together in Figure 2. According to Figure, there are some areas of the pressure–temperature curve where the equilibrium pressure for the creation of CH4 hydrates is higher than that for the formation of CO2 hydrates at the same temperature. This explains why the CO2–CH4 exchange process occurs because CO2 hydrates are more stable than CH4 hydrates under these circumstances.
Between 2011 and 2012, a field test (Ignik Sikumi Gas Hydrate Exchange Field Experiment) was conducted in the Prudhoe Bay Unit on the Alaska North Slope. The methane hydrate reservoir beneath the permafrost was filled with a CO2/N2 combination. The flowback data showed that there was a “bulk exchange” between the injected gas and CH4; however, it is unclear whether this happened through the direct CO2–CH4 exchange process or through some other mechanism. The exothermic nature of the CO2 hydrate synthesis creates heat during the CH4-CO2 exchange to encourage CH4 hydrate dissociation.
[5] CO2 storage as a hydrate in depleted gas fields
One of the main alternatives for traditional geological sequestration of CO2 is the storage of CO2 in exhausted oil and gas sources. The production of CO2 hydrates in those fields that are partially saturated with water under the right temperature and pressure circumstances may improve the long-term stability of CO2 storage. The most important variables in determining possible locations with conditions conducive to CO2 hydrate production and long-term stability are the geothermal and pressure data of these reservoirs. Porosity, permeability, size, geochemistry, hydrodynamic regime, closeness, accessibility, and other characteristics are also crucial for reservoirs. The majority of the research done so far on this subject has been on the Canadian province of Alberta, where the mean annual ground surface temperature frequently falls below 7 °C. According to the calculations, Alberta’s depleted gas reservoir can hold about 46 Gt of CO2.
The efforts were done as four distinct injection schemes in the lab, including gas cap injection, top spiral tube injection, bottom spiral tube injection, and double spiral tube injection, to explore the injection of pure CO2 into partial water-saturated (25% water saturation) porous media. This study showed that the development of CO2 hydrates can be encouraged by a well-designed method.
The gas hydrate research group under the guidance of Prof. Rajnish Kumar has been doing active research to study carbon dioxide sequestration using gas hydrate technology at IIT Madras. The research involved the experimental study from a 50 ml setup to a 10000 ml pilot scale setup in which the condition below the seabed is mimicked in order to conduct the experiments on CO2 sequestration and CO2 – CH4 exchange. A detailed understanding of the impact of chemical additives on CO2 gas hydrate has been developed to optimize the technology. In another approach, molecular dynamics based simulation is also performed to explore the interaction at the molecular level during the CO2 gas hydrate formation and dissociation. For further information, please go through the latest research publications of our group (https://www.researchgate.net/profile/Rajnish-Kumar-40).
Author – @Bhavik Mahant, Prime Minister Research Fellow, Ph.D. Scholar, Gas Hydrate Research Group, Department of Chemical Engineering, Indian Institute of Technology Madras



